CHANG TSI
Insights
The fourth amendment to the Patent Law of the People’s Republic of China (hereinafter, the “Patent Law”) came into effect on June 1, 2021. In July of the same year, the Measures for the Implementation of the Early Resolution Mechanism for Drug Patent Disputes (Trial), together with corresponding judicial and administrative adjudication rules, were issued and brought into force. These developments marked the official launch of China’s early resolution mechanism for pharmaceutical patent disputes (also known as the pharmaceutical patent linkage system, hereafter, the “Patent Linkage System” or the “Early Resolution Mechanism”). As of the date of writing, the system has been in operation for more than four years.
This series of articles examines China’s patent linkage system. It outlines the legislative background and key features of the regime and, through collation and analysis of relevant case data, summarizes the adjudication principles that emerged in practice for readers’ reference.
A. What Is the Patent Linkage System?
The patent linkage system is a mechanism that “links” the patent status of a drug to the regulatory review and approval process for its marketing authorization.
The intent of the system is that, through institutional design, potential patent infringement disputes are resolved before a generic drug is approved, thereby balancing two policy objectives: facilitating timely market entry of generics and safeguarding the patent rights of original drugs.
Under this mechanism, when submitting a marketing authorization application, a generic drug applicant must make a formal declaration regarding any relevant patents. If a dispute arises, the patent holder of the original drug or the interested party may, during the review stage, bring a civil lawsuit or apply for an administrative adjudication to determine whether the generic drug falls within the scope of the relevant patent. Pending a court judgment or administrative adjudication decision, the drug regulatory authority may suspend the approval process for the generic drug.
B. Legal Basis and Regulatory Development
The legal foundation for the patent linkage system in China is set out in Article 76 of the Patent Law, as revised in 2020.
Article 76 states that, during the drug review and approval process, if a dispute arises on the grounds that the drug applied for involves another party’s patent rights, the parties may file a lawsuit in a people’s court or request an administrative adjudication from the China National Intellectual Property Administration (hereinafter, “CNIPA”). The drug regulatory authority (the National Medical Products Administration, hereinafter, “NMPA”) may, based on an effective court judgment, decide whether to suspend the approval process for the drug’s marketing authorization.
To implement Article 76, the NMPA and CNIPA jointly issued in 2021 the Measures for the Implementation of the Early Resolution Mechanism for Drug Patent Disputes (Trial) (hereinafter, the Measures for the Implementation). In parallel, CNIPA issued the Measures for Administrative Adjudication of the Early Resolution Mechanism for Drug Patent Disputes (hereinafter, the Measures for Administrative Adjudication), providing procedural guidance for the handling of patent linkage cases.
In addition, the Provisions of the Supreme People’s Court on Several Issues Concerning the Application of Law in the Trial of Civil Cases of Patent Right Disputes Related to Drug Registration Application (hereinafter, the “Judicial Interpretation on Civil Cases of Drug Patent Disputes”) serve as an important legal reference for judicial proceedings.
C. Case Acceptance
1. Administrative Adjudication
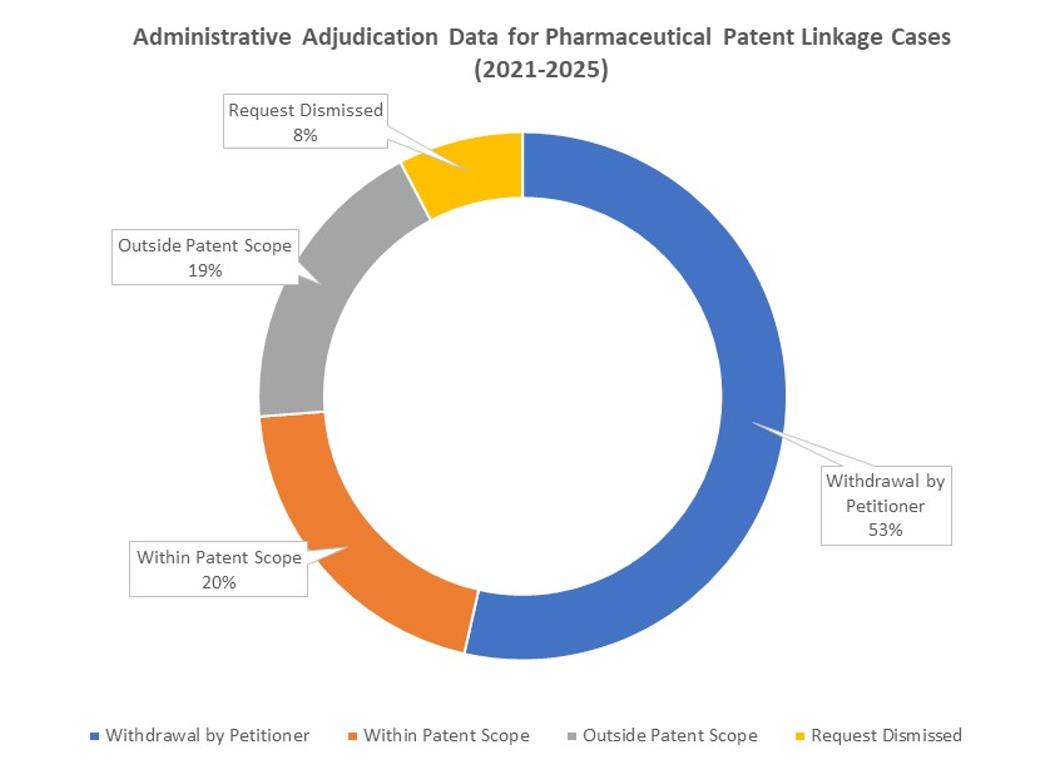
As of November 14th, 2025, CNIPA had published 183 administrative adjudication decisions involving pharmaceutical patents. More than half of these cases (98 cases) were voluntarily withdrawn by the petitioner.
Of the cases adjudicated on the merits:
2. Civil Judgments and Rulings
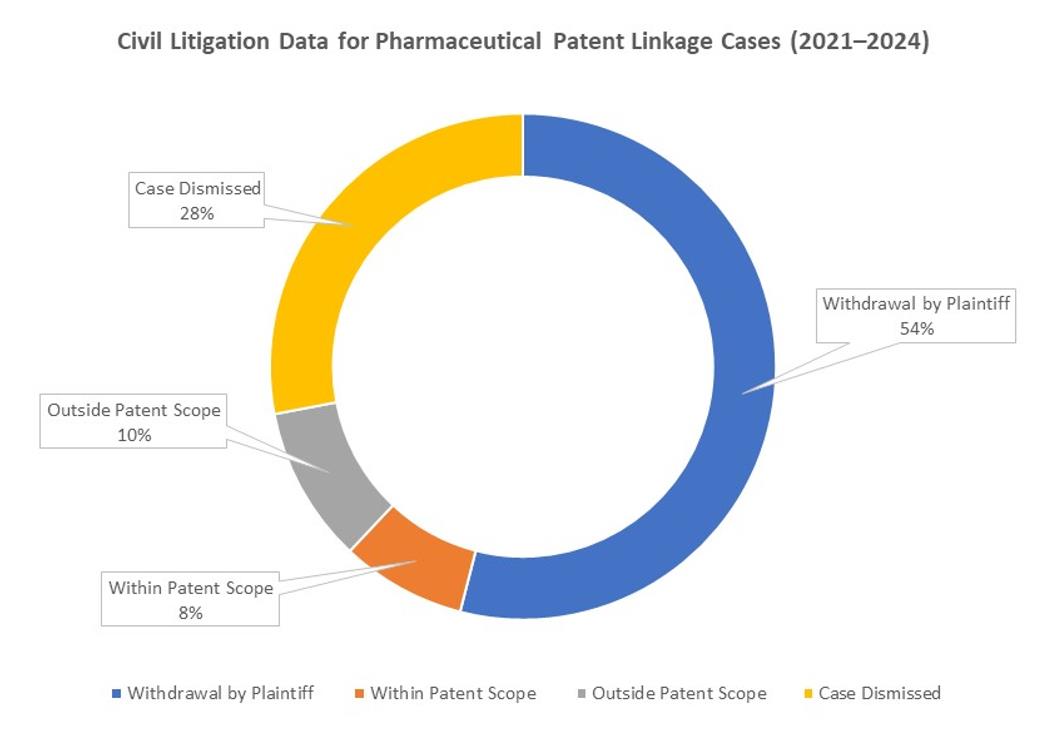
As of the same date, China Judgments Online and major legal databases recorded 50 civil judgments and rulings related to the patent linkage system, comprising 32 first-instance cases and 18 second-instance cases.
In approximately 54% of these cases, the plaintiff voluntarily withdrew the action during the proceedings. Of the remainder, 14 were dismissed at the filing stage, and only 9 proceeded to substantive trial, ultimately resulting in court judgments.
A. Criteria for Application of the Early Resolution Mechanism
The Early Resolution Mechanism can only be invoked where certain prerequisites are satisfied. Based on the relevant provisions of the Patent Law and currently available administrative adjudication decisions and civil judgments, the principal criteria may be summarized as follows:
In addition, during substantive examination of the case, the court/CNIPA may also address the following specific issues:
The marketing authorization holder (MAH) must register the relevant patent information for a drug approved and marketed in China on the Patent Information Registration Platform for Marketed Drugs.
“Relevant patent information” must meet the following criteria:
Further, the registered patent must be of a type eligible for listing under the Measures for the Implementation.
B. Procedural Framework of the Early Resolution Mechanism
1. Patent Information Registration by the MAH
Article 4 of the Measures for the Implementation requires that, to trigger the mechanism, the MAH must register the relevant patent information within 30 days of obtaining the drug registration certificate. If any registered patent information changes, the MAH must update the platform within 30 days of the change taking effect.
The platform conducts only a formal examination. The MAH is responsible for the authenticity, accuracy, and completeness of the submitted information, which must remain consistent with:
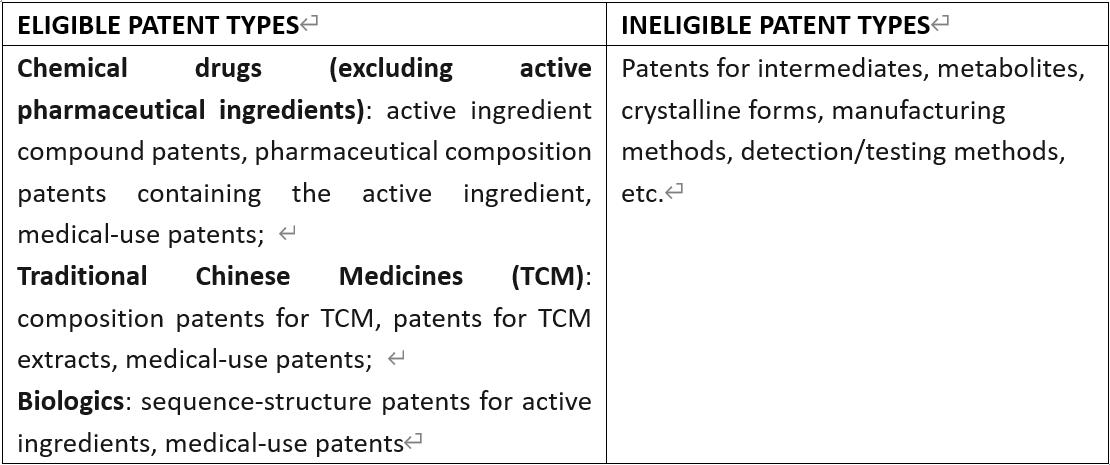
Pursuant to the Measures for the Implementation and relevant Policy Interpretation, the scope of patent types eligible/ineligible for registration is as follows:
2. Generic Drug Applicant’s Declaration
Articles 6 and 12 of the Measures for the Implementation require that applicants for chemical generics, same-name/same-formula TCM products, and biosimilars, when submitting a marketing authorization application, must review the patent information disclosed on the Patent Information Registration Platform for Marketed Drugs, and make a declaration for each pharmaceutical patent relating to the reference drug.
The four declaration categories are:
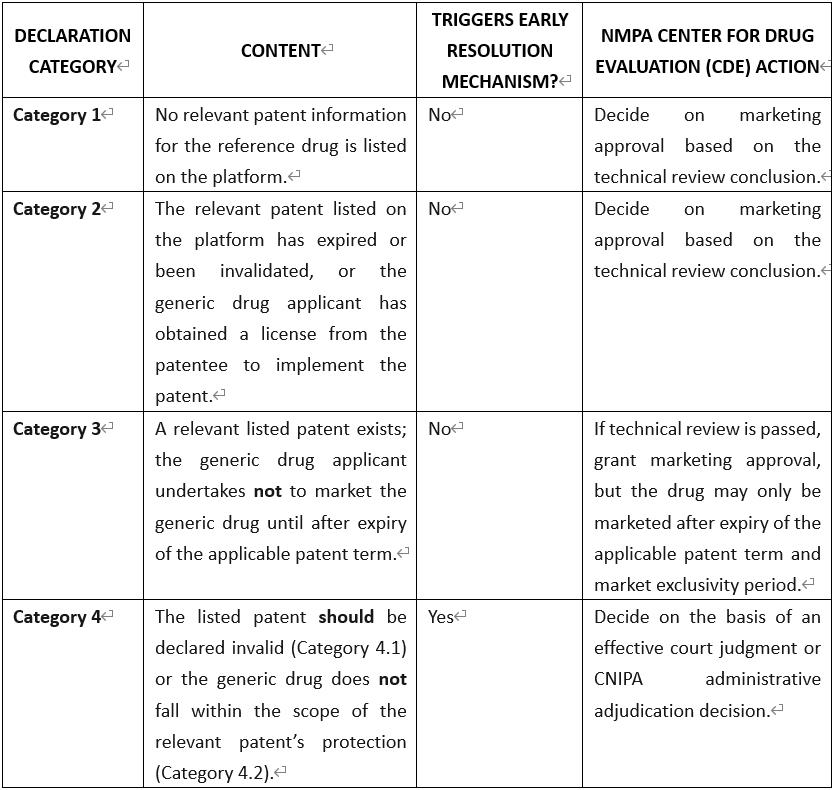
After a generic drug applicant makes a declaration, the CDE must, within 10 working days, publicly disclose the application information and the declaration on the information platform.
The generic drug applicant bears responsibility for the truthfulness and accuracy of the declaration, and must notify the MAH of the reference drug; where the MAH is not the patentee, the MAH must in turn notify the patentee.
Where a declaration asserts that the drug does not fall within the protection scope of the relevant patent, the supporting materials must include:
Nevertheless, in (2023) CNIPA YaoCai No.0007, the CNIPA held that although a generic drug applicant’s failure to fulfil the information-exchange obligation under Article 6 of the Measures for the Implementation was culpable, where compliance with the information-exchange requirement was unconnected to determining whether the generic drug’s technical solution fell within the patent scope, a request to compel compliance did not fall within the scope of an administrative adjudication. Pursuant to this adjudication decision, a claim in which the generic drug applicant is requested to amend the statement category, or to discharge its obligation to notify the MAH of the statement and its basis, is not likely to be sustained by the CNIPA.
3. Early Resolution Civil Action / Administrative Adjudication Initiated by Patentee or an Interested Party / Generic Drug Applicant
Following publication of the generic marketing authorization application, within 45 days of the CDE’s public disclosure, the patentee or an interested party (i.e., a licensee of the relevant patent or the registered MAH of the reference drug) may, if objecting to a category 4 declaration, file a lawsuit in the people’s court or request an administrative adjudication from CNIPA.
Moreover, If the patentee or the interested party fails to commence court proceedings within the 45-day period, pursuant to Article 4 of the Judicial Interpretation on Civil Cases of Drug Patent Disputes, a drug marketing authorization applicant (a generic drug applicant) may either file a lawsuit with a people’s court or request the CNIPA to render an administrative adjudication in relation to a drug patent dispute, seeking a determination that the drug for which registration is being applied does not fall within the scope of protection of the relevant patent right.
The Measures for the Implementation specify that CNIPA will not accept a drug patent dispute case if a people’s court has already docketed the matter. However, the Judicial Interpretation on Civil Cases of Drug Patent Disputes provides that where a party cites CNIPA’s acceptance of an administrative adjudication request under Article 76 of the Patent Law as a reason to argue that the court should not accept a lawsuit, or should suspend it, the court will not support that position.

4. Waiting Period and Outcomes
Upon receiving a copy of the case-filing notice from the court or the notice of acceptance from the CNIPA, as submitted by the parties to the case, the drug regulatory authority (the NMPA) will impose a 9-month waiting period on the registration application for a chemical generic drug.
The waiting period shall commence on the date of case filing by the court or acceptance by the CNIPA, and may be imposed only once. During this period, the CDE will continue its technical review without suspending the evaluation process. The CDE will proceed as follows based on the outcome of the court or CNIPA adjudication decision:
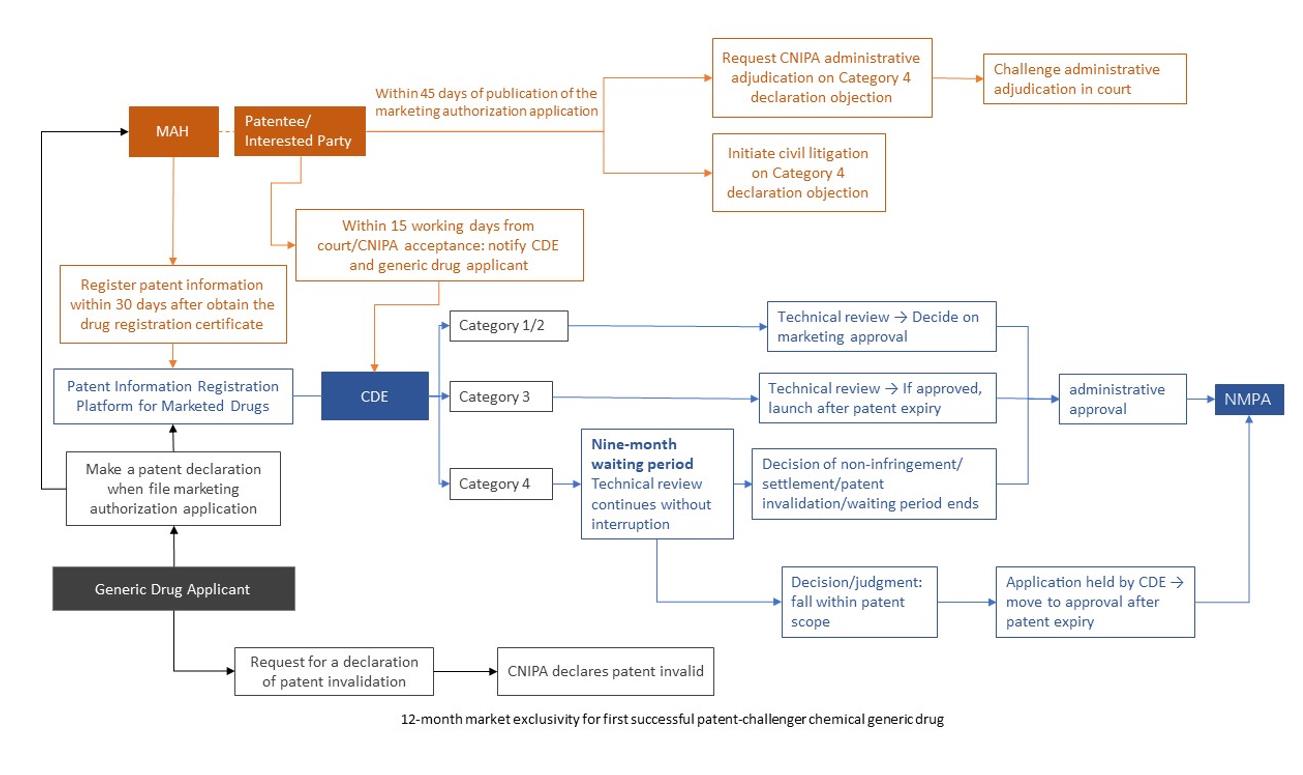
Additionally, applications for registration of same-name/same-formula TCMs and biosimilars are not subject to the waiting period. The CDE will directly determine whether to grant marketing approval. However, if a court or the CNIPA confirms that the relevant technical solution falls within the scope of the relevant patent’s protection, the generic drug may only be marketed after the expiry of the corresponding patent term.
The above provides an overview of China’s pharmaceutical patent linkage system, including the criteria for its application and its core procedural framework. In our next article, we will explore more details of the specific rules for patent linkage cases and other practical issues.


